You are not using a standards-compliant web browser. This site looks strikingly better if you use a standards-compliant web browser. However, this site is still functional with your browser.
This is a short list of standards-complaint browsers that will make your visit to this site a more pleasurable one: Mozilla Firefox, Netscape 7, Opera 6 or greater, or Internet Explorer 5 or greater.
Information for
Related Organizations

THE ROLE OF AGRICULTURE IN
NITROUS OXIDE EMISSIONS
Nitrous
oxide (N2O), the third most abundant greenhouse gas, is closely tied
to production agriculture. Agriculture activities accounted for 76 percent of N2O
emissions in the
These
activities include the application of organic and inorganic fertilizers,
planting nitrogen-fixing crops, burning plant residue, managing manure, and
adding nitrogen to water sources from field runoff, according to the U.S.
Environmental Protection Agency.
|
Emissions from agriculture (Tg CO2 Eq.) |
||||||||
|
Year |
1990 |
1995 |
2000 |
2001 |
2002 |
2003 |
2004 |
2005 |
|
N2O |
375.9 |
362.7 |
386.9 |
399.2 |
376.2 |
359.9 |
348.7 |
375.1 |
|
Agriculture soil management |
366.9 |
353.4 |
376.8 |
389.0 |
366.1 |
350.2 |
338.8 |
365.1 |
|
Manure management |
8.6 |
9.0 |
9.6 |
9.8 |
9.7 |
9.3 |
9.4 |
9.5 |
|
Field burning of agriculture residue |
0.4 |
0.4 |
0.5 |
0.5 |
0.4 |
0.4 |
0.5 |
0.5 |
Source: http://epa.gov/climatechange/emissions/downloads06/07Agriculture.pdf
Much
attention has been targeted toward carbon dioxide, which is the most abundant
greenhouse gas in the atmosphere; however, N2O is about 300 times
more powerful due to its duration in the atmosphere and its heat-absorbing
capabilities.
Each
greenhouse gas is given a carbon equivalent, or a value of global warming
potential. Carbon dioxide is the baseline of this scale with a value of one.
Methane has a value of 21, and N2O has a value of 310, making it much
more effective at warming the atmosphere.
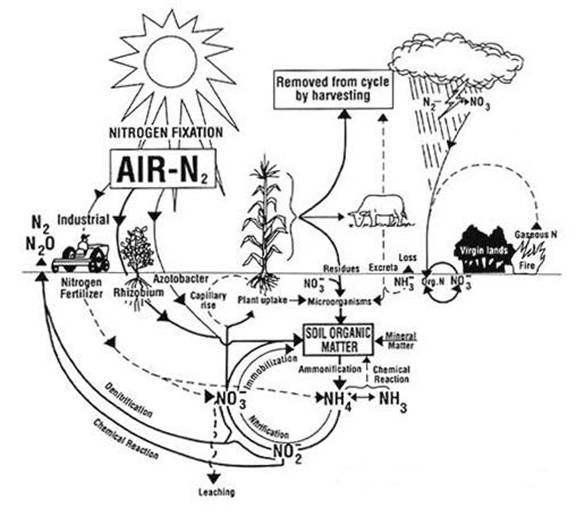
Nitrogen
naturally enters the soil through the activities of nitrogen-fixing bacteria
(and blue-green algae), and the deposition of animal wastes. Even if there were
no human activity, these processes would occur and convert inert atmospheric
nitrogen into forms that are essential to plant and animal life. Nitrogen-fixing
bacteria absorb atmospheric nitrogen and transform it into ammonia (NH3). Some
nitrogen-fixing bacteria are free-living in soil and water, while others are
symbiotic with certain plants, fungi, and other organisms. The NH3 produced by
symbiotic bacteria is rapidly converted into certain amino acids within the
plants. Once this nitrogen is incorporated into plant tissue, it then cycles
back into the soil as organic residue. As organic matter is decomposed, the
nitrogen is transformed into ammonium (NH4) and simple organic compounds. The
NH4 then goes through the microbial-driven processes of nitrification and
denitrification.
Nitrification and denitrification are driven by the activity of
microorganisms in soils. Nitrification is the aerobic microbial oxidation of
ammonium (NH4) to nitrate (NO3). In a well-aerated soil,
most of the ammonium in the soil is converted into nitrate. Denitrification is the anaerobic microbial reduction of
nitrate to nitrogen gas (N2). Nitrous
oxide is a gaseous intermediate product in the reaction sequence of
denitrification, which leaks from microbial cells into the soil and then into
the atmosphere. Nitrous oxide is also produced during nitrification, although
by a less well-understood mechanism.
Nitrogen
also enters the soil as a direct result of human activities, and this has a
significant indirect effect on nitrous oxide emissions. Nitrogen additions
through human activities are done mainly through organic and inorganic
fertilization. Both sources of nitrogen fertilizer lead to increased levels of
mineral and organic nitrogen in the soil, which lead to higher rates of
nitrification and possibly denitrification. Also, the planting of
nitrogen-fixing crops such as soybean and alfalfa is a human activity that
increases the natural process of nitrogen fixation in the soil. Burning crop
residues also contributes to N2O emissions due to the incomplete
combustion of agricultural waste, but occurs on a much smaller scale than
fertilizer applications.
Studies
are underway to understand how agricultural and land-use management practices
can affect N2O emissions, but this has not yet been extensively
researched. Manure management, for example, plays a factor in nitrous oxide
emissions, but methane has been researched more intensively than N2O
on this subject. Research has shown that inorganic nitrogen application
practices can have a significant effect on N2O emissions, but more
work is needed on this topic.
An
indirect source of N2O emissions is from water leaching and runoff
from agricultural fields. Applied nitrogen fertilizer and manure that is not
absorbed by crops may leach through the soil or wash into surface waters. There,
part of the nitrogen is converted into N2O through denitrification.
Some
believe that water runoff is a major contribution to N2O emissions,
but little research has been done to determine that.
Nitrous
oxide emissions vary greatly from region to region, depending on agricultural
production and waste management practices, climate, soil type, and
transportation factors. For example, temperate, intensive agricultural areas
such as the Midwestern U.S. will produce much more nitrous oxide than other areas
because of the quantities of nitrogen that are added to the soil.
In
summary, potential nitrous oxide mitigation practices in agriculture include:
*
Improved nitrogen fertilizer efficiency
*
Improved nitrogen placement, timing, and rate
*
Reduced soil erosion
-- Katie Starzec, CASMGS
Communications,