
SOIL
CARBON AND CLIMATE CHANGE NEWS
From
Consortium for Agricultural Soils
Mitigation of Greenhouse Gases (CASMGS)
http://soilcarboncenter.k-state.edu
Charles W. Rice, K-State Department of
Agronomy, National CASMGS Director
(785) 532-7217 cwrice@ksu.edu
Scott Staggenborg, K-State Department of
Agronomy (785) 532-7214 sstaggen@ksu.edu
Steve Watson, CASMGS Communications (785)
532-7105 swatson@ksu.edu
November 30, 2007
No. 59
Science and Research:
* Modeling Nitrous Oxide Emissions In Agriculture
* The Role Of Agriculture In
Nitrous Oxide Emissions
* Measuring Soil Carbon
Rapidly With Near-Infrared Spectroscopy
International:
*
New Voluntary Carbon Standard Released
**********
MODELING
NITROUS OXIDE
EMISSIONS
IN AGRICULTURE
Developing and utilizing
accurate computer Decision Support Systems (DSS) for nitrous oxide (N2O)
emissions from soils could help predict how various land use management
practices would affect emissions of this greenhouse gas. William Salas, president
of Applied Geosolutions, LLC is developing a geospatial DSS for N2O emissions,
utilizing an assortment of digital data, research data, and a biogeochemical
model. The DSS is being used estimate and map nitrous oxide emissions at
various site levels, and the potential effect of land management practices on
these emissions. Salas is also working on methods for scaling up the results to
extrapolate from site scale to watershed scale and beyond.
The biogeochemical model
being used by Salas is the process-oriented DNDC
(DeNitrification-DeComposition), developed by Changseng Li, of the
The core DNDC model is based
on biogeochemical concepts for predicting soil carbon and nitrogen fluxes. The
model links the impact of specified ecological drivers (climate, topography,
soil, vegetation, and anthropogenic activity) on carbon, nitrogen, and water
cycles. All three elemental cycles (C, N, and water) are linked through a
biogeochemical field that includes radiation, temperature, moisture, pH, Eh,
and substrate gradients.
Salas is using the DNDC
Biogeochemical Process model in combination with GIS-based spatial information
to produce the NUGGET-DNDC data mapping tool -- a web-based, GIS-based Decision
Support System for predicting the fate of C and N in a given site.
Salas first incorporates
data on climate, soils, land use cropland management practices, and hydrology.
He uses a variety of climate data in developing the NUGGET-DNDC mapping tool,
including DAYMET, NEXRAD, and NCDC station data for the
Once this basic data have
been compiled for a given site, the next step is to take into account the
effect of different agricultural management practices (using field research
data). This information is then processed through the DNDC model, which
simulates the carbon and nitrogen cycles and incorporates the biological and
physical factors that affect those cycles. The final analysis is subject to
whatever sensitivity analysis is desired, and a product is generated.
The final product is a map
of the site (such as a watershed) showing a predicted range of possible nitrous
oxide emissions at each point in the site as a result of changes in management
practices. The NUGGET-DNDC Decision Support System is being used to assess the
impact of agricultural management practices on the release of carbon and
nitrogen to air and water. The goal is to be able to develop site-specific best
management practices for increasing soil carbon and reducing nitrogen emissions
and losses in a given site.
NUGGET-DNDC was developed
with support from the USDA Small Business Innovative Research program.
Background on the
NUGGET-DNDC mapping tool can be found at http://www.appliedgeosolutions.com.
For information on the DNDC models, check out the “Resources” section of that
web site. Li’s DNDC model web page is at: http://dndc.sr.unh.edu
For more information on the
NUGGET-DNDC mapping tool, contact William Salas at wsalas@agsemail.com.
-- Steve Watson, Editor
**********
THE ROLE OF AGRICULTURE IN
NITROUS OXIDE EMISSIONS
Nitrous
oxide (N2O), the third most abundant greenhouse gas, is closely tied
to production agriculture. Agriculture activities accounted for 76 percent of N2O
emissions in the
These
activities include the application of organic and inorganic fertilizers,
planting nitrogen-fixing crops, burning plant residue, managing manure, and
adding nitrogen to water sources from field runoff, according to the U.S.
Environmental Protection Agency.
|
Emissions from agriculture (Tg CO2 Eq.) |
||||||||
|
Year |
1990 |
1995 |
2000 |
2001 |
2002 |
2003 |
2004 |
2005 |
|
N2O |
375.9 |
362.7 |
386.9 |
399.2 |
376.2 |
359.9 |
348.7 |
375.1 |
|
Agriculture soil management |
366.9 |
353.4 |
376.8 |
389.0 |
366.1 |
350.2 |
338.8 |
365.1 |
|
Manure management |
8.6 |
9.0 |
9.6 |
9.8 |
9.7 |
9.3 |
9.4 |
9.5 |
|
Field burning of agriculture residue |
0.4 |
0.4 |
0.5 |
0.5 |
0.4 |
0.4 |
0.5 |
0.5 |
Source: http://epa.gov/climatechange/emissions/downloads06/07Agriculture.pdf
Much
attention has been targeted toward carbon dioxide, which is the most abundant
greenhouse gas in the atmosphere; however, N2O is about 300 times
more powerful due to its duration in the atmosphere and its heat-absorbing
capabilities.
Each
greenhouse gas is given a carbon equivalent, or a value of global warming
potential. Carbon dioxide is the baseline of this scale with a value of one.
Methane has a value of 21, and N2O has a value of 310, making it much
more effective at warming the atmosphere.
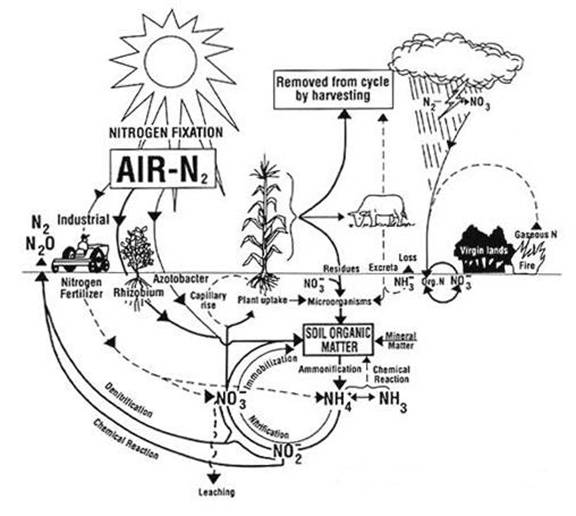
Nitrogen
naturally enters the soil through the activities of nitrogen-fixing bacteria
(and blue-green algae), and the deposition of animal wastes. Even if there were
no human activity, these processes would occur and convert inert atmospheric
nitrogen into forms that are essential to plant and animal life. Nitrogen-fixing
bacteria absorb atmospheric nitrogen and transform it into ammonia (NH3). Some
nitrogen-fixing bacteria are free-living in soil and water, while others are
symbiotic with certain plants, fungi, and other organisms. The NH3 produced by
symbiotic bacteria is rapidly converted into certain amino acids within the
plants. Once this nitrogen is incorporated into plant tissue, it then cycles
back into the soil as organic residue. As organic matter is decomposed, the
nitrogen is transformed into ammonium (NH4) and simple organic compounds. The
NH4 then goes through the microbial-driven processes of nitrification and
denitrification.
Nitrification and denitrification are driven by the activity of
microorganisms in soils. Nitrification is the aerobic microbial oxidation of
ammonium (NH4) to nitrate (NO3). In a well-aerated soil,
most of the ammonium in the soil is converted into nitrate. Denitrification is the anaerobic microbial reduction of
nitrate to nitrogen gas (N2). Nitrous
oxide is a gaseous intermediate product in the reaction sequence of
denitrification, which leaks from microbial cells into the soil and then into
the atmosphere. Nitrous oxide is also produced during nitrification, although
by a less well-understood mechanism.
Nitrogen
also enters the soil as a direct result of human activities, and this has a
significant indirect effect on nitrous oxide emissions. Nitrogen additions
through human activities are done mainly through organic and inorganic
fertilization. Both sources of nitrogen fertilizer lead to increased levels of
mineral and organic nitrogen in the soil, which lead to higher rates of
nitrification and possibly denitrification. Also, the planting of
nitrogen-fixing crops such as soybean and alfalfa is a human activity that
increases the natural process of nitrogen fixation in the soil. Burning crop
residues also contributes to N2O emissions due to the incomplete
combustion of agricultural waste, but occurs on a much smaller scale than
fertilizer applications.
Studies
are underway to understand how agricultural and land-use management practices
can affect N2O emissions, but this has not yet been extensively
researched. Manure management, for example, plays a factor in nitrous oxide
emissions, but methane has been researched more intensively than N2O
on this subject. Research has shown that inorganic nitrogen application
practices can have a significant effect on N2O emissions, but more
work is needed on this topic.
An
indirect source of N2O emissions is from water leaching and runoff
from agricultural fields. Applied nitrogen fertilizer and manure that is not
absorbed by crops may leach through the soil or wash into surface waters. There,
part of the nitrogen is converted into N2O through denitrification.
Some
believe that water runoff is a major contribution to N2O emissions,
but little research has been done to determine that.
Nitrous
oxide emissions vary greatly from region to region, depending on agricultural
production and waste management practices, climate, soil type, and
transportation factors. For example, temperate, intensive agricultural areas
such as the Midwestern U.S. will produce much more nitrous oxide than other areas
because of the quantities of nitrogen that are added to the soil.
In
summary, potential nitrous oxide mitigation practices in agriculture include:
*
Improved nitrogen fertilizer efficiency
*
Improved nitrogen placement, timing, and rate
*
Reduced soil erosion
-- Katie Starzec, CASMGS
Communications,
http://ohioline.osu.edu/aex-fact/0463.html
**********
Measuring Soil Carbon Rapidly With
Near-Infrared Spectroscopy
An
emerging method of soil carbon measurement involves a tractor and lightwaves.
The technology is called near-infrared spectroscopy (NIR). Veris Technologies,
based in
The
process involves a light that is directed at the soil through a sapphire
window. The window is pressed directly against the soil and is pulled 5 cm
below the soil surface. The light bounces off the soil, and is broken into
wavelengths like a prism. Those wavelengths can be calibrated to produce a
carbon map of the field. A companion NIR probe is used to measure soil carbon
up to 60 cm below the soil surface to get carbon measurements at a wide range
of depths.
The
advantages are that hundreds of carbon measurements can be taken at a time, and
no soil preparation is needed, says Eric Lund of Veris Technologies.
Near-infrared
light waves are between visible light waves and microwaves on the
electromagnetic spectrum. NIR spectroscopy has been used since the 1950s to
test grains, feeds, meat, and other biological materials. It has also been used
in the pharmaceutical industry because of its nondestructive nature, according
to a presentation titled “Mapping Soil
Carbon with On-The-Go Near Infrared Spectroscopy” by Colin Christy of
Veris Technologies
(http://www.oznet.ksu.edu/ctec/Fall%20Forum%20pdf%20files/Papers_Abstracts/Christy_Veris.pdf). Portable pull-behind NIR technology for
in-field measurements has been pursued since the 1980s.
NIR
takes carbon measurements, says
For
maximum accuracy, this should be done on a field-to-field basis -- and in the
future, an area-to-area basis, says
-- Katie Starzec, CASMGS
Communications,

Figure 1. The NIR equipment from Veris Technologies making field measurements using a shank.

Figure 2. To calibrate the NIR equipment readings, soil probes are first used to determine actual soil carbon levels.
**********
New
Voluntary Carbon
Standard
Released
The Climate Group, the
International Emissions Trading Association (IETA), and the World Business
Council for Sustainable Development (WBCSD) launched a new global carbon offset
standard at the London Stock Exchange on Nov. 20, 2007 to increase
participation and confidence in the global voluntary carbon market.
The Voluntary Carbon Standard (VCS) provides a quality assurance check for
voluntary offset projects, and is designed to complement and support other
credible approaches to verifying carbon emission reduction practices. The transparency
of the new VCS is designed to boost market confidence in the voluntary carbon
market.
Market analysts estimate that annual transactions in the voluntary carbon
market could reach $4 billion in the next five years and that the VCS - already
popular with buyers - will be instrumental to this future growth, according to
the Voluntary Carbon Standard Association.
For more details on the
Voluntary Carbon Standard, see:
http://www.v-c-s.org/news.html
For specific information on
how the standard applies to agricultural land, see page 20-25 of the following publication
“Voluntary Carbon Standard: Guidance for Agriculture, Forestry, and Other Land
Use Projects”:
http://www.v-c-s.org/docs/AFOLU%20Guidance%20Document.pdf
For an overview of the
voluntary carbon markets and the different standards being proposed for this
market, see Soil Carbon and Climate Change News No. 57, at:
http://soilcarboncenter.k-state.edu/newsletters/09_18_07.htm
-- Steve Watson, Editor
swatson@ksu.edu
**********
MEETINGS OF INTEREST
Dec. 17-18, 2007
CASMGS Forum: Agriculture's
Role in the New Carbon Economy
http://soilcarboncenter.k-state.edu/Fall_Forum_CASMGS.html
**********
Send comments or items for the newsletter
to Steve Watson at:
NOTE: If you are forwarding this
newsletter to someone who would like to
subscribe on their own, here's how they
can do so:
To subscribe:
Send a message to <mailserv@lists.oznet.ksu.edu>
Skip the Subject line
in the body of the message, type:
<subscribe carbon>
Then hit the return key twice.
If you would like to remove your name from
this list and no longer
receive this newsletter, here's how:
To unsubscribe:
Send a message to <mailserv@lists.oznet.ksu.edu>
Skip the Subject line
in the body of the message, type:
<unsubscribe carbon>
Then hit the return key twice.